Anhydrous Ammonia - Overview

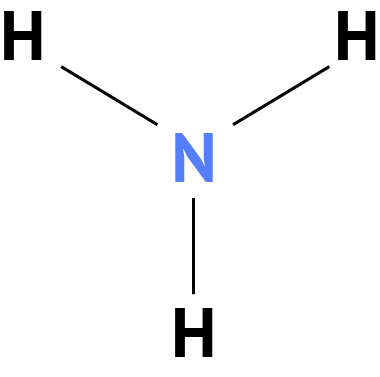
Ammonia, sometimes called Azane, is a compound with the formula NH3. It is normally encountered as a gas with a characteristic pungent odor. Ammonia contributes significantly to the nutritional needs of the planet as a precursor to foodstuffs and fertilizers. Ammonia, either directly or indirectly, is a building block for the synthesis of many pharmaceuticals. Although it is in wide use, ammonia is caustic and hazardous.
Ammonia used commercially is usually named anhydrous ammonia. This term emphasizes the absence of water. Because NH3 boils at -33 °C, the liquid must be stored under pressure or at low temperature. Its heat of vaporization is, however, sufficiently high that NH3 can be readily handled in ordinary beakers in a fume hood. “Household ammonia” or “ammonium hydroxide” is a solution of NH3 in water. The strength of such solutions is measured in units of baume (density), with 26 degrees baume (about 30 weight percent ammonia at 15.5 °C) being the typical high concentration commercial product. Household ammonia ranges in concentration from 5 to 10 weight percent ammonia.
Anhydrous Ammonia Properties
Ammonia is a colorless gas with a characteristic pungent smell similar to human urine, as the urine contains an amount of ammonia in it. It is lighter than air, its density being 0.589 times that of air. It is easily liquefied due to the strong hydrogen bonding between molecules; the liquid boils at -33.3 °C, and solidifies at -77.7 °C to a mass of white crystals. Liquid ammonia possesses strong ionizing powers (ε = 22), and solutions of salts in liquid ammonia have been much studied. Liquid ammonia has a very high standard enthalpy change of vaporization (23.35 kJ/mol, cf. water 40.65 kJ/mol, methane 8.19 kJ/mol, phosphine 14.6 kJ/mol) and can therefore be used in laboratories in non-insulated vessels at room temperature, even though it is well above its boiling point. It is miscible with water. All the ammonia contained in an aqueous solution of the gas may be expelled by boiling. The aqueous solution of ammonia is basic. The maximum concentration of ammonia in water (a saturated solution) has a density of 0.880 g /cm³ and is often known as ‘.880 Ammonia’. Ammonia does not burn readily or sustain combustion, except under narrow fuel to air mixtures from 15-25% air. When mixed with oxygen, it burns with a pale yellowish-green flame. At high temperature and in the presence of a suitable catalyst, ammonia is decomposed into its constituent elements. Chlorine catches fire when passed into ammonia, forming nitrogen and hydrochloric acid; unless the ammonia is present in excess, the highly explosive nitrogen trichloride (NCl3) is also formed.
Manufacturing Process
Mass production of Ammonia mostly uses the Haber–Bosch process, reacting hydrogen (H2) and nitrogen (N2) at a moderately-elevated temperature (450 °C) and high pressure (100 standard atmospheres (10,000 kPa))

The Uses of Anhydrous Ammonia
Ammonia used commercially is usually named anhydrous ammonia. This term emphasizes the absence of water. Because NH3 boils at -33 °C, the liquid must be stored under pressure or at low temperature. Its heat of vaporization is, however, sufficiently high that NH3 can be readily handled in ordinary beakers in a fume hood. “Household ammonia” or “ammonium hydroxide” is a solution of NH3 in water. The strength of such solutions is measured in units of baume (density), with 26 degrees baume (about 30 weight percent ammonia at 15.5 °C) being the typical high concentration commercial product. Household ammonia ranges in concentration from 5 to 10 weight percent ammonia.
Agriculture Industry
In addition to serving as a fertilizer ingredient, ammonia can also be used directly as a fertilizer by forming a solution with irrigation water, without additional chemical processing. This later use allows the continuous growing of nitrogen dependent crops such as maize (corn) without crop rotation but this type of use leads to poor soil health.
Refrigeration
Ammonia’s thermodynamic properties made it one of the refrigerants commonly used in refrigeration units prior to the discovery of dichlorodifluoromethane. In 1928, also known as Freon or R12.
But ammonia is toxic, gaseous, irritant, and corrosive to copper alloys, and over a kilo is needed for even a miniature fridge. With an ammonia refrigerant, the ever present risk of an escape brings with it a risk to life. However data on ammonia escapes has shown this to be an extremely small risk in practice, and there is consequently no control on the use of ammonia refrigeration in densely populated areas and buildings in almost all jurisdictions in the world.
Disinfectant
It is also sometimes added to drinking water along with chlorine to form chloramine, a disinfectant. Unlike chlorine on its own, chloramine does not combine with organic (carbon containing) materials to form carcinogenic halomethanes such as chloroform. However, chlorine and ammonia should never be mixed in an uncontrolled environment because they cause a chemical reaction that releases toxic gas. See Safety precautions for more information.
Food Industry
Ammonia is used in food industry as a source of nitrogen for fermentation process and pH adjuster
Chemical Manufacturing
In chemical manufacturing, ammonia is used as a precursor to nitrogenous compounds
Hazards Identification
Hazard Warnings: Anhydrous ammonia is a flammable which can causes severe skin burns and eye damage. It is a toxic gas which is dangerous if inhaled. It also very toxic to aquatic environment.


Precautionary Statements: Obtain special Instructions before use If exposed or concerned, get medical advice/attention Do not handle until all safety precautions have been read and understood. Wear protective gloves, eye protection. Dispose of contents/container to comply with local, state and federal regulations.
